Kinases and their Dependence on Chaperones
Kinases in all eukaryotes are dependent on Hsp90, its cofactors and other chaperones. But about 50% of the kinases do not require Hsp90. We aim at understanding, what defines an Hsp90-dependent kinase and which structural changes make the differences. To this aim we use the Caenorhabditis elegans and the mammalian Hsp90-system togeth with its cofactor Cdc37.
The chaperone Hsp90 is a molecular machine, which utilizes ATP hydrolysis to induce conformational changes in its client kinase. We are interested in the mechnism of these reactions. To this aim we analyze the nature of these conformational changes and the interaction of Hsp90 with Cdc37 and its partner kinases.
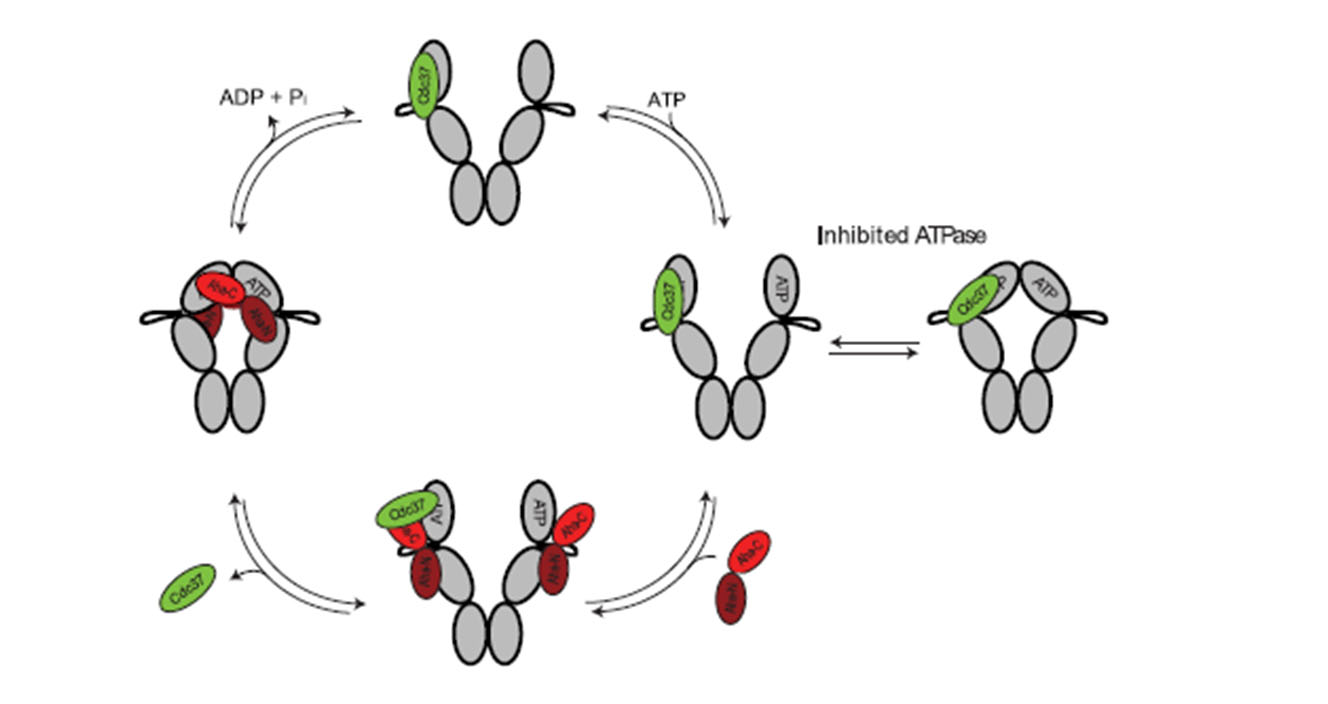
Hsp90 cycle and the regulation of conformations by co-factors
Also the regulation by other cofactors (Aha1, p23, STI-1, PPH-5, UNC-45 und weitere) is under investigation. Using protein chemical methods, like ITC, SPR-spectroscopy, FRET and analytical ultracentrifugation next to enzymatic assays. Using the model organism C. elegans we also investigate steps during the development to a multi-cellular nematode, which require the function of Hsp90 or its client proteins. These processes are during the gonad development, the muscle development and the regulation of the heat shock response